WHY DOES THE PERIODIC TABLE LOOK THE WAY IT DOES?
Good question! Well first let’s look at the periodic table and how it works.
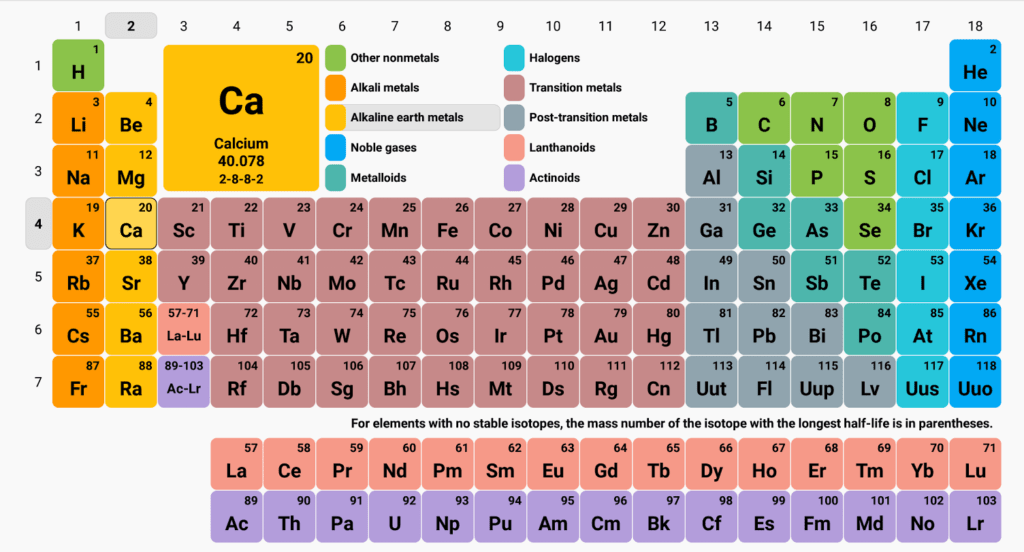
WHAT DOES THE PERIODIC TABLE SHOW?
The periodic table contains all of the elements we have ever had or created on earth. Elements after 95 (and also elements 43 and 61) are not naturally found on earth and have to be made in the lab. If you’ve forgotten what elements are then check out my post on them!
HOW ARE THE ELEMENTS ORDERED? IS IT RANDOM?
No it isn’t random at all. Take a look back at the periodic table. If you go from left to right along the rows you’ll see the top number (the atomic number) goes up by one every time (of course when you finish one row go back to the start of another).
WHY DO SOME ROWS HAVE LESS ELEMENTS THAN OTHERS?
Remember when we talked about electrons we talked about shells? Well each shell has a certain capacity. Once full, any new electrons have to make a new shell. Different shells have different capacities.
The first row of the periodic table has 2 elements, the first electron shell has a capacity of 2 electrons. The next 2 rows have 8 elements, the next 2 shells have a capacity of 8 electrons and it goes on as the rows in the periodic table get wider and wider.
For the exam you’ll need to know how to write out the electron configuration for elements. Basically you just split up the electrons into their shells. So aluminum for example has 13 electrons. The first shell fits 2 which leaves us with 11. The second shell fits 8 electrons, so far we have 2, 8. After filling the first 2 shells we have a remainder of 3 electrons which take spaces in the next shell. So the electron configuration of aluminum is 2, 8, 3. It’s easy right?
Once again I hope this was helpful, let me know if what and what I need to do to make it better down in the comments! If you have a question or topic you’re stuck on email me (go to contact page). Thanks!