SYLLABUS STATEMENTS 3.1 - 3.8, 5.6 - 5.9, 5.13 - 5.21
Hey I’ve recently uploaded a new video to my YouTube channel on Organic Chemistry.
But some things in this topic I thought it would be easier to understand in writing instead of in video.
HOMOLOGOUS SERIES
A homologous series is basically a series of compounds with the same general formula. Alkanes and Alkenes are homologous series’, they have the general formula CnH2n+2 and CnH2nrespectively.
NAMING ALKANES AND ALKENES
So basically for this you need to know that:
meth – 1 carbon
eth – 2 carbons
prop – 3 carbons
but – 4 carbons
pent – 5 carbons
So you have a hydrocarbon. To name it first look at the number of carbons.
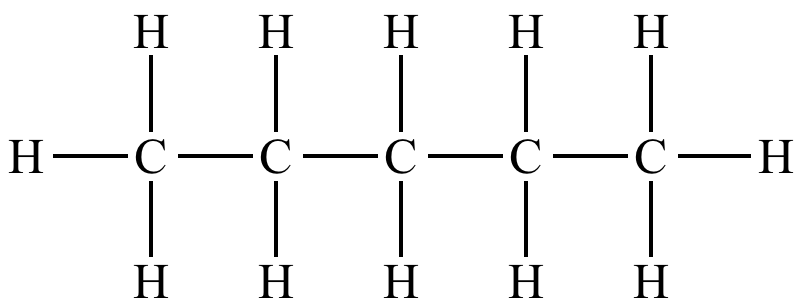
So here we have 5 carbons, so the name starts with ‘pent’. Next look at which homologous series it is from (is it an alkane or an alkene?). This molecule is an alkane so the name ends in ‘ane’. So put those two parts together and we have pentane.
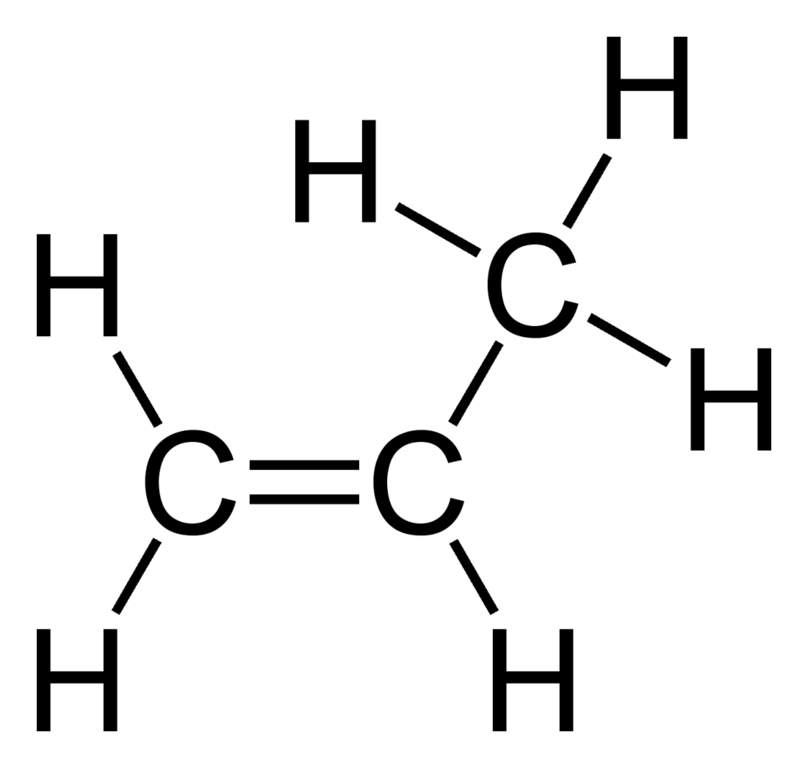
Here we have another molecule. First look at the number of carbons. Here we have 3 carbons so the first part of the name is ‘prop’. Next figure out which homologous series it is from (is it an alkane or alkene?). This molecule is an alkene (double bond) so the end of the name is ‘ene’. Put those 2 together and we have propene.
BROMINE METHANE SUBSTITUTION REACTION
So a bit like how I mentioned in the plastics part of my video, you can add other elements as a substitute for the hydrogens on a hydrocarbons. Of course as soon as you add an element other than Carbon and Hydrogen, the molecule is no longer a hydrocarbon. You can substitute one of the hydrogens in methane with a bromine in a reaction. One condition for this reaction though, UV light.
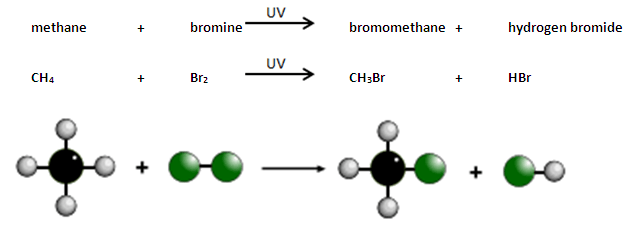
Well why the UV light? Well this is not something you need to know but I find it quite interesting. Normally, Bromine atoms will pair up (because Halogens are diatomic). When they are paired up they are quite content on their own and are not interested in reacting with methane. They UV light has just the right amount of energy to break the bonds in the bromine molecule, creating what is called free radicals. These bromine radicals react with the methane molecules to make bromomethane.
CRACKING
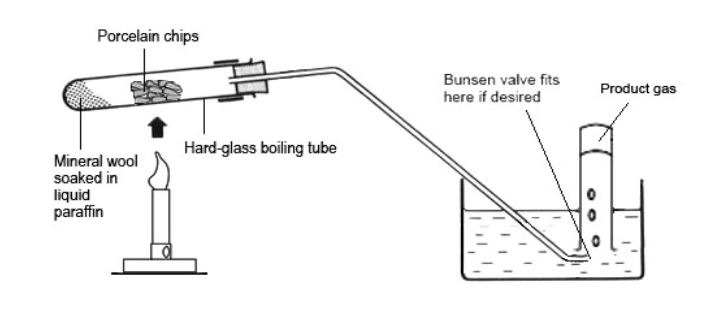
From this process we get short chain hydrocarbons which tend to be gases. For this cracking to occur, we need heat and a catalyst. The catalyst in this reaction is aluminium oxide or porous pot. The liquid paraffin vapourises and passes over the hot catalyst where it decomposes into shorter chain hydrocarbons.
ONE LAST THING.
You need to remember that addition polymers (explained in video) do not biodegrade easily as they are relatively inert.
THAT'S IT!
That’s everything you need to know about organic chemistry! Once again I hope this has been useful to you. If not, comment what I need to improve and I’ll get to it!