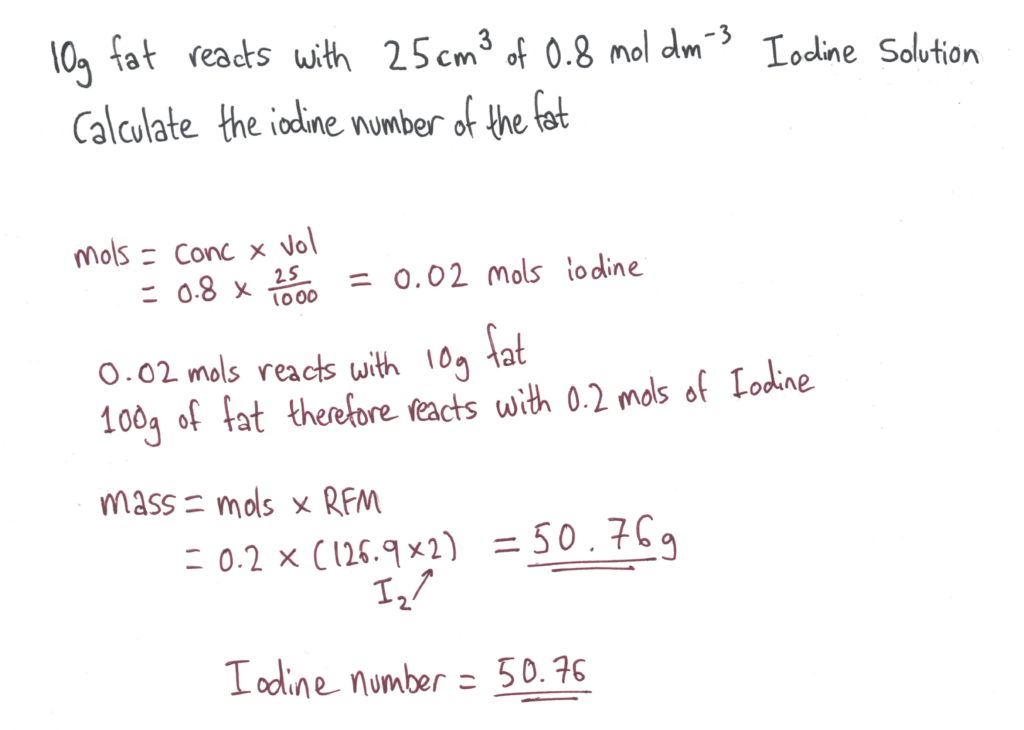What are lipids?
Lipids act as structural components of cell membranes, in energy storage, thermal and electrical insulation, as transporters of lipid soluble vitamins and as hormones.
Fats are more reduced than carbohydrates and so yield more energy when oxidized.
Comparison of carbohydrates and lipids as energy storage molecules with respect to their solubility and energy density.
Lipids are basically what the common person would call fats, although it’s not necessarily that simple.
- Largely non polar
- And therefore insoluble.
- A great energy source (better than carbs
)
- More reduced than carbs.
- Work as chemical messengers (hormones)
- Absorb certain vitamins and minerals
- Used for electrical and thermal insulation
- Make up cell membranes
Although lipids have a much higher energy density than carbohydrates, they are not used in the body as a source of immediate energy because they are insoluble in blood where energy needs to be carried.
Lipids have 3 main forms:
- Triglycerides
- Phospholipids
- Steroids
Fatty Acids
Fatty acids can be saturated, monounsaturated or polyunsaturated.
Prediction of the relative melting points of fats and oils from their structures.
Fatty acids are effectively just really long carboxylic acids.
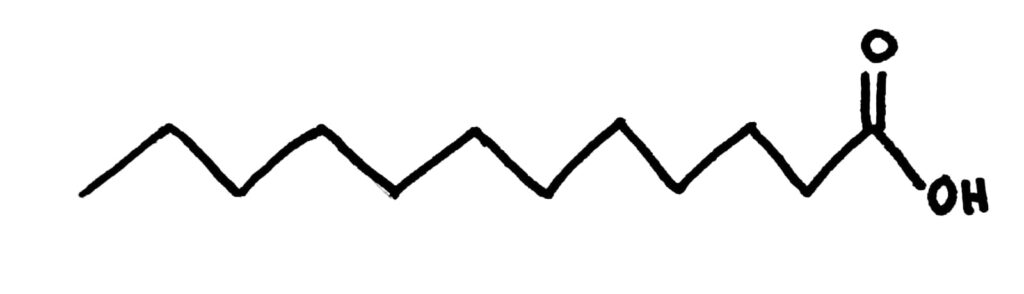
A saturated fatty acid
Fatty acids can, like other molecules with long carbon chains, be saturated or unsaturated, with unsaturated being split into monounsaturated and polyunsaturated. Monounsaturated fatty acids have only a single double bond while polyunsaturated fatty acids have multiple double bonds.
Cis and Trans fats
As we’ve learnt in organic chem, double bonds can be either cis or trans. This is the same in fatty acids and it changes their properties.
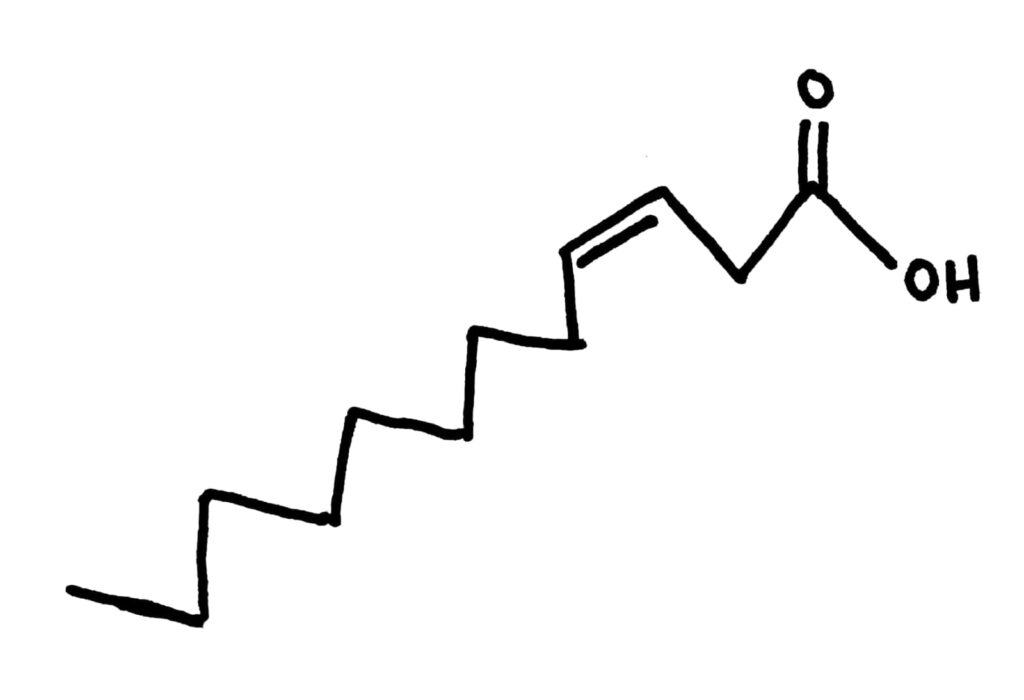
Cis fatty acids have a kink in their chain, giving them a kind of weird shape. This shape means that they don’t pack together as well, and usually have a low boiling point because of decreased LDFs compared to trans fatty acids. They’re usually liquid at room temperature. Most natural fats and oils are cis 
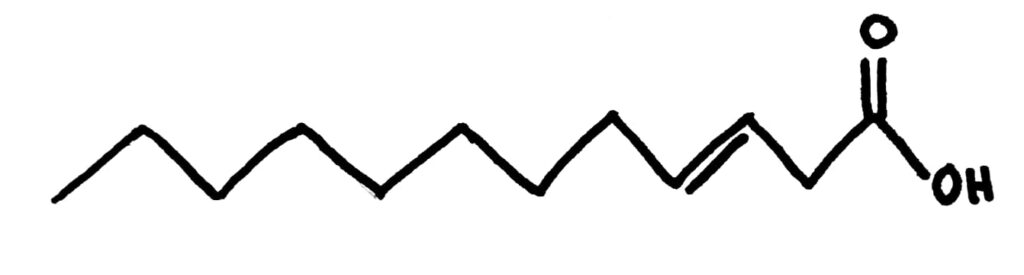


Trans fatty acids definitely pack much better than cis fatty acids, you can see it just from the number of them I managed to fit in the space where just 1 cis fatty acid could fit. This gives them a higher melting point because they also have increased LDFs compared to cis fatty acids and means they’re usually solids at room temperature 
Synthetic fats commonly have more trans double bonds (due to the hydrogenation process), and evidence points towards them giving people high blood pressure and heart disease. This is because they pack together and block arteries, causing high blood pressure.
Triglycerides
Triglycerides are produced by condensation of glycerol with three fatty acids and contain ester links.
Deduction of the structural formulas of reactants and products in condensation and hydrolysis reactions between glycerol and fatty acids and/or phosphate.
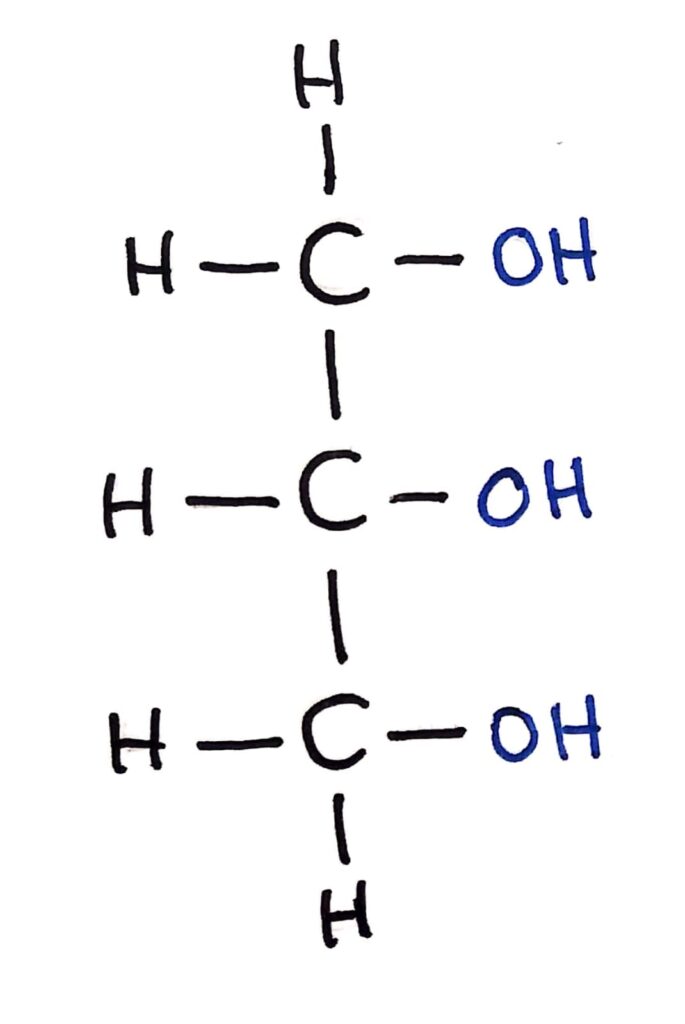
Triglycerides are all based around one molecule which acts as their ‘backbone’, glycerol. Glycerol of course isn’t the IUPAC name for it though, it’s actually propan-1,2,3-triol.
Fatty acids bond to the OH group of glycerol in an esterification reaction to form triglycerides. This reaction is a condensation reaction, because 3 water molecules are made.
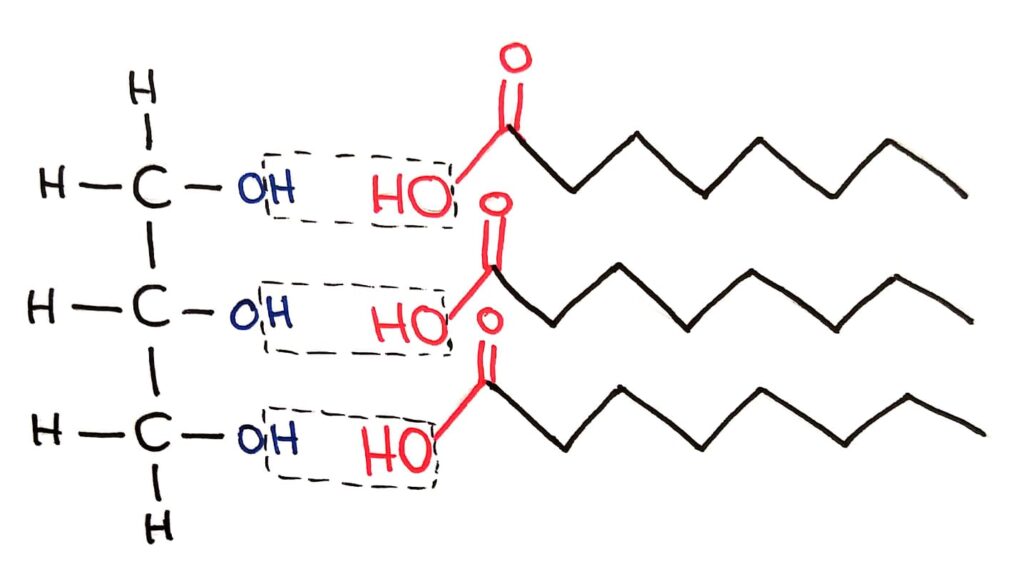
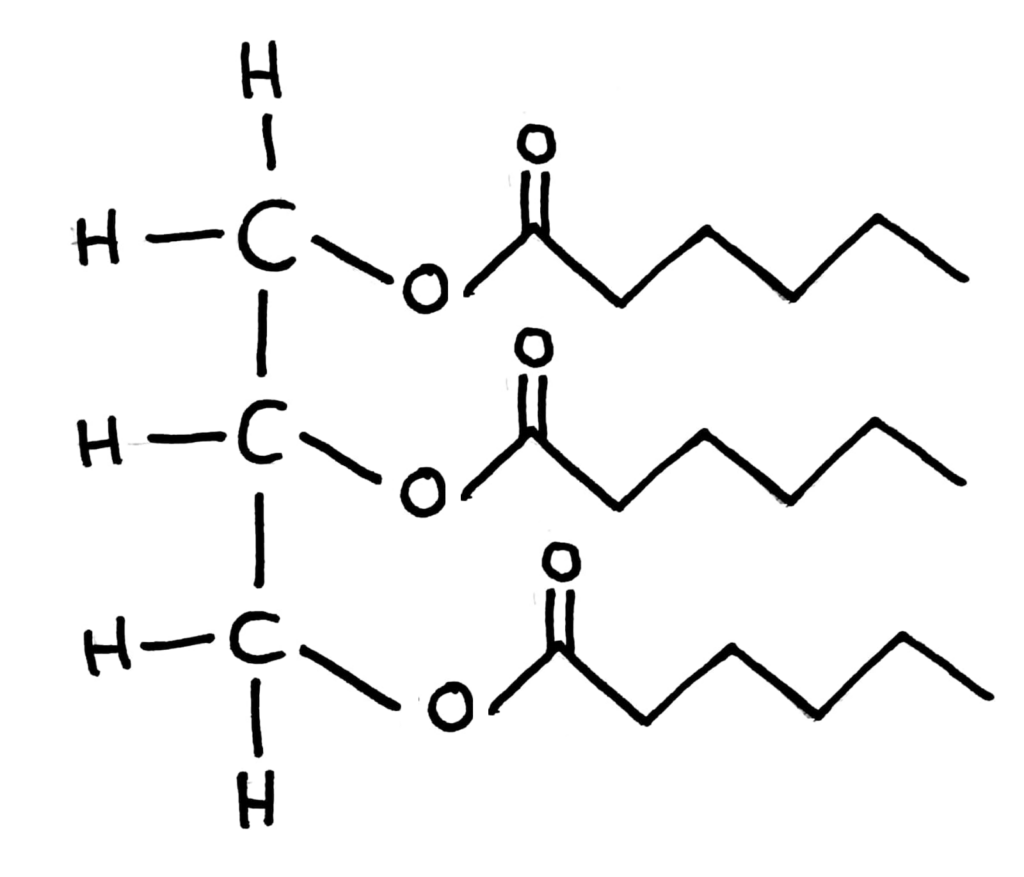
+ 3H2O
Melting points of triglycerides
Prediction of the relative melting points of fats and oils from their structures.
The melting points of triglycerides is affected by:
- Fatty acid chain length
- Saturation
- Saturated, mono or polyunsaturated
- Packing
- London Dispersion forces, more double bonds mean less hydrogens
- Saturated, mono or polyunsaturated
- Cis trans isomers
- Packs together better
Phospholipids
Phospholipids are derivatives of triglycerides.
Phospholipids are essentially the same as triglycerides, except one fatty acid chain is replaced by a phosphate group on the glycerol molecule. This addition of the phosphate group gives phospholipids cool properties.
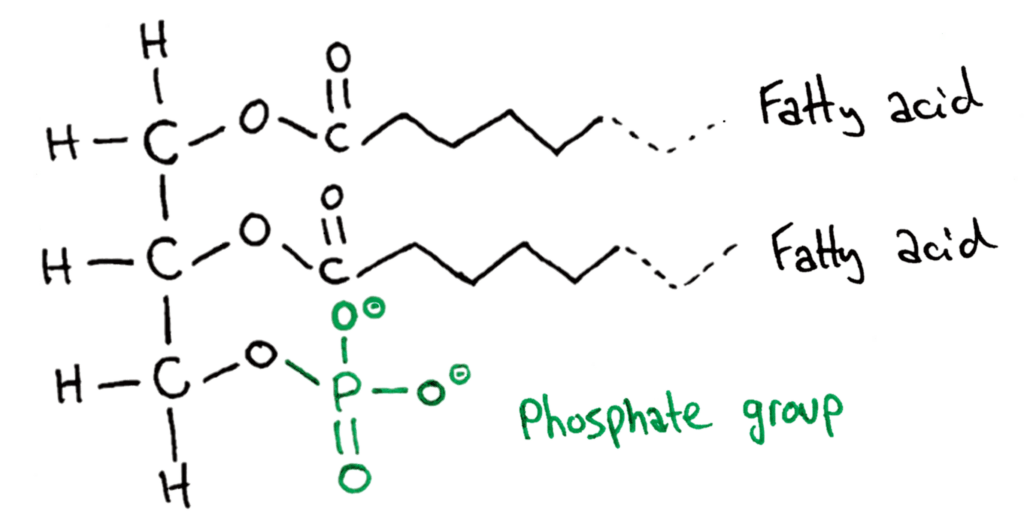
Because this phosphate group has been added to the structure, it now has a polar and non-polar part of the molecule. The phosphate group is very polar, and the fatty acid chains are very non polar.
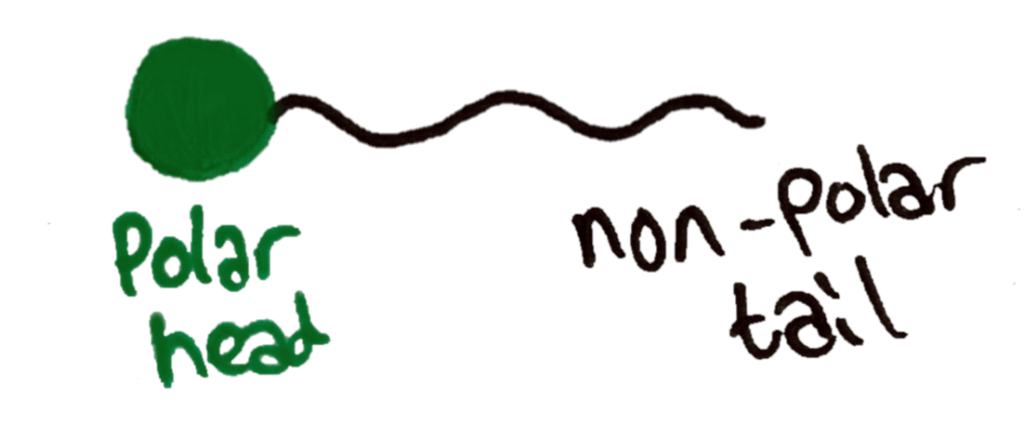
In water, phospholipids align themselves in certain ways due to their polar and non-polar parts. While their polar parts remain soluble thanks to the phosphate groups, their non polar part is attracted to other non polar molecules.
In the blood, phospholipids can surround insoluble vitamins like A,D,E and K and make them soluble by forming a structure called a micelle
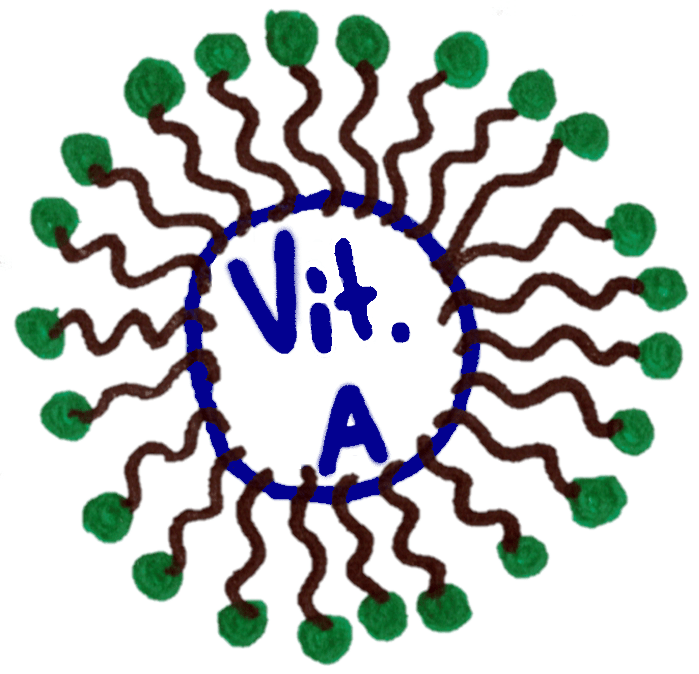
Imagine this structure but in 3D, with phospholipids forming a ball around the insoluble vitamin
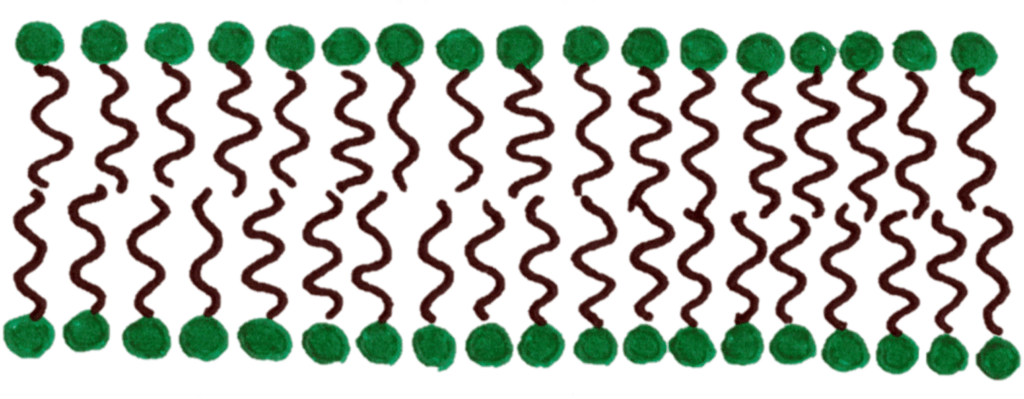
Phospholipids are also used in cell membranes to form the phospholipid bilayer. The phospholipids polar and non polar groups align to form a layer that is polar on the outside.
Hydrolysis
Hydrolysis of triglycerides and phospholipids can occur using enzymes or in alkaline or acidic conditions.
Acid Hydrolysis
In acid hydrolysis, triglycerides are split into glycerol and 3 fatty acids in acidic conditions (strong acid).
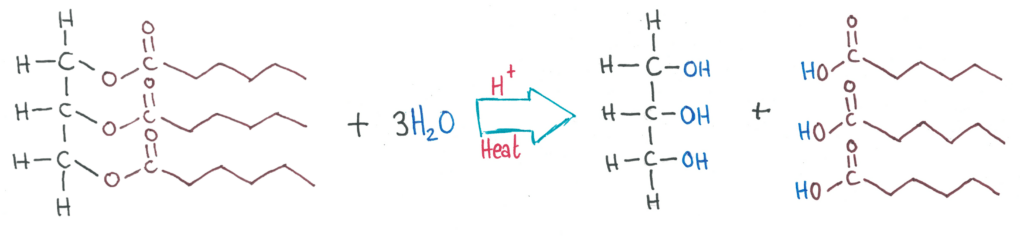
Alkaline Hydrolysis
Alkaline hydrolysis splits triglycerides into glycerol and the salts of the fatty acid chains. The salts of fatty acid chains are actually the main components of soaps, and this reaction is actually often called a saponification reaction.
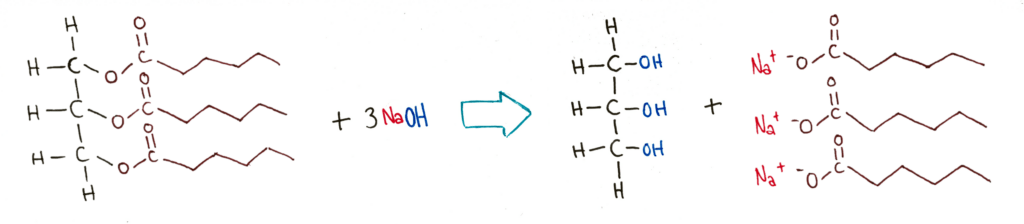
Hydrolysis of Phospholipids
All you need to know about this is that when phospholipids hydrolyse, the phosphate group is not affected.
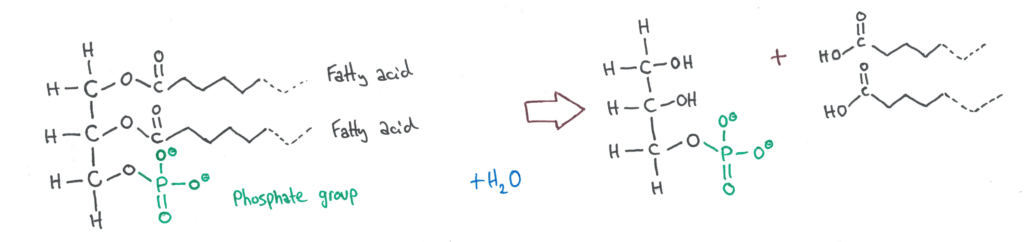
Steriods
hehe
Steroids have a characteristic fused ring structure, known as a steroidal backbone.
Discussion of the impact of lipids on health, including the roles of dietary high- density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, saturated, unsaturated and trans-fat and the use and abuse of steroids.
Steroids are a class of molecules that are based around a fused ring structure, the 4 ring steroidal backbone.
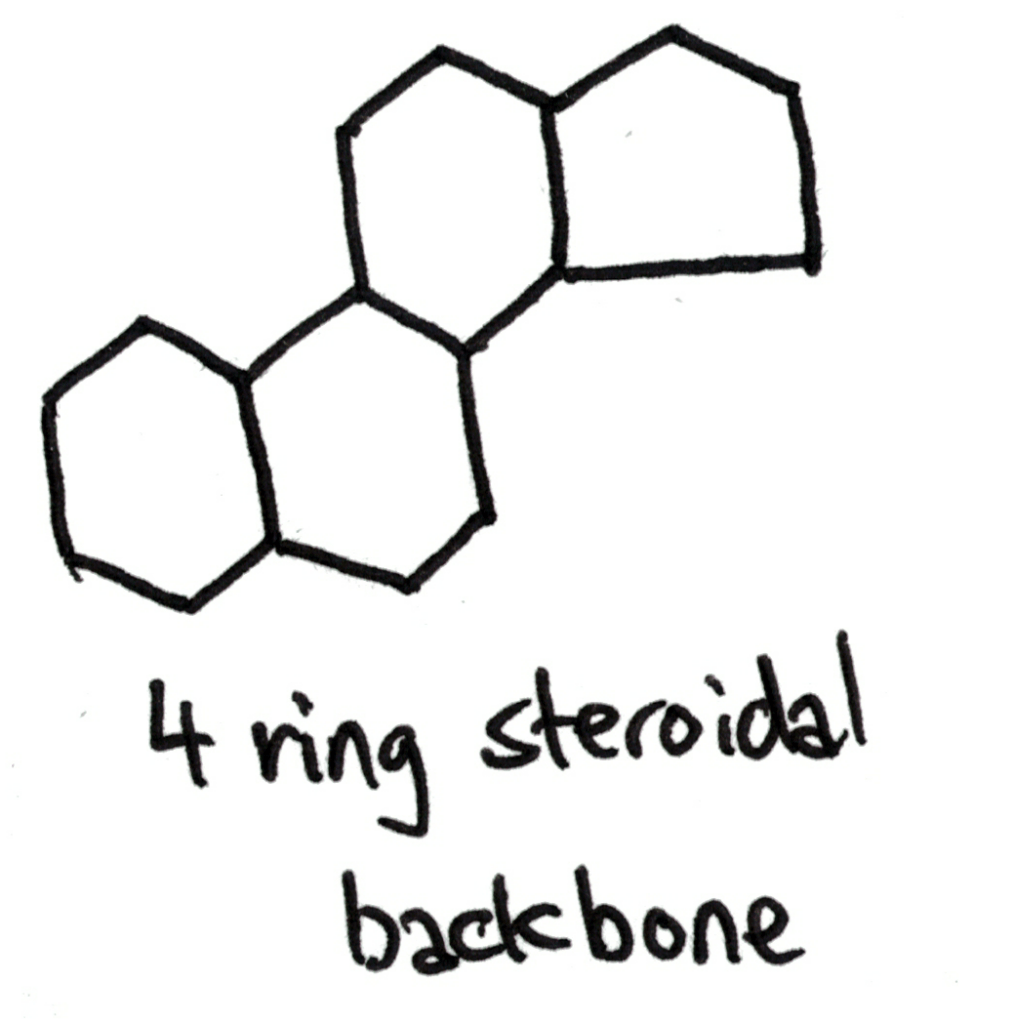
Steroids are actually naturally produced in the body and have a lot of important uses:
- Used as a structural component of cells
- A lot of steroids are hormones
Examples of steroids in the body:
- Testosterone
- Estrogen and Progesterone
- Cholesterol
Cholesterol
There are 2 different types of Cholesterol, LDL (low density lipoprotein) and HDL (high density lipoprotein). LDL is harmful to health because it can block arteries and cause heart disease and strokes. HDL is healthy though, it actually removes LDL from arteries so HDL is the MVP.
Steroid Abuse 
- Testosterone in sports
- To increase strength
- Damages male reproductive organs
- Men can develop breasts?!?!
Rancidity of fats and oils
Comparison of the processes of hydrolytic and oxidative rancidity in fats with respect to the site of reactivity in the molecules and the conditions that favour the reaction.
Hydrolytic Rancidity
Hydrolytic rancidity is effectively just hydrolysis of triglycerides into glycerol and fatty acids.
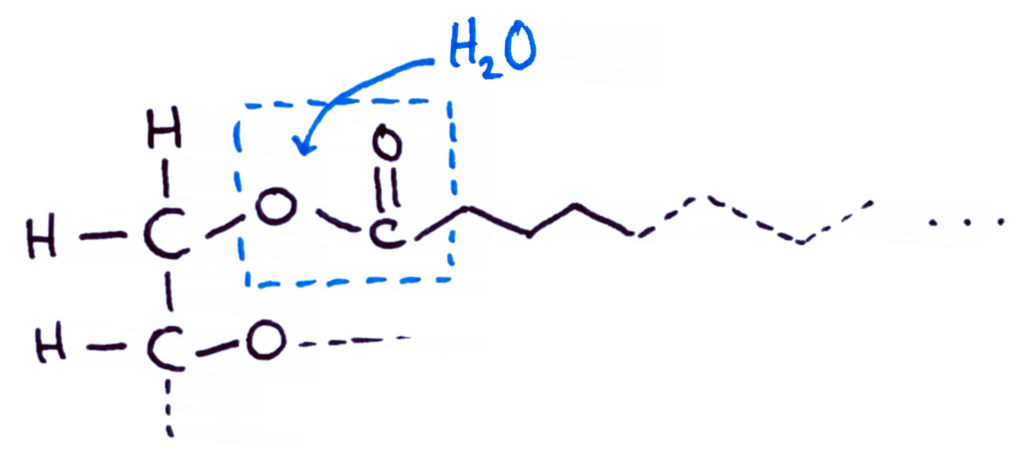
Water is able to break the ester bond between the glycerol and the fatty acid, breaking down the triglyceride.
Increased rate caused by:
- More water
- Higher Temperature
- Presence of enzymes
Oxidative Rancidity
Oxidative rancidity is where oxygen reacts with double bonds in the fat to produce ketones and aldehydes. Oxygen reacts with the fat with the help of light in a free radical reaction.
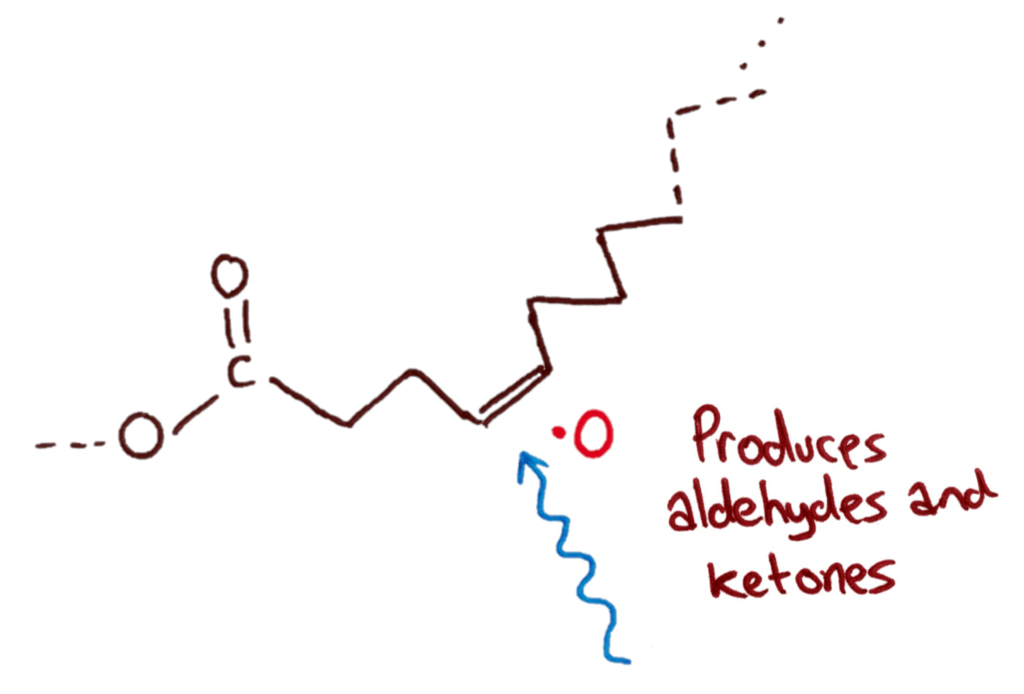
Rate increased by:
- Presence of oxygen
- Light
- Heat
Oxidative rancidity often occurs in fish 
Iodine Number
Application of the concept of iodine number to determine the unsaturation of a fat.
The Iodine Number of a fat or oil is the mass of iodine that reacts with 100g of the fat.
Iodine reacts across the double bonds in the fat or oil, making it a suitable reaction to test the degree of unsaturation of a fat or oil.
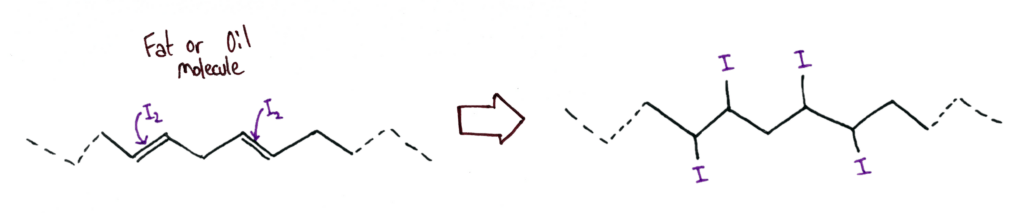
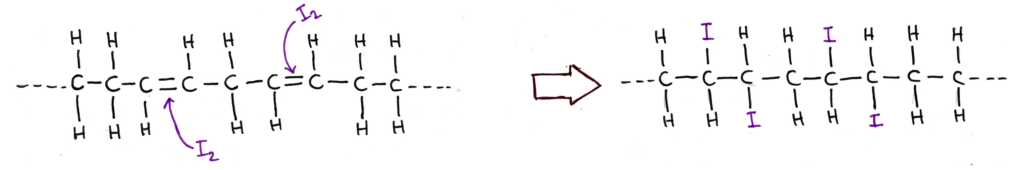
It’s a useful way to qualitatively express the degree of unsaturation of a fat compared to just labelling fats and oils as polyunsaturated, which could mean it has 2 double bonds or 100 double bonds.
The Experiment
Iodine is titrated slowly into a sample of the fat or oil and decolourises from dark brown/purple to colourless as it reacts with the fat, making it a self indicating reaction.
When the iodine no longer decolourises, the reaction is complete and the iodine number can be calculated like a titration calc: