B.10 - Stereochemistry in Biomolecules
Amino Acids
All amino acids (except for 1 exception) are chiral have two different optical isomers. (Click here for a recap on what they are from 20.3).
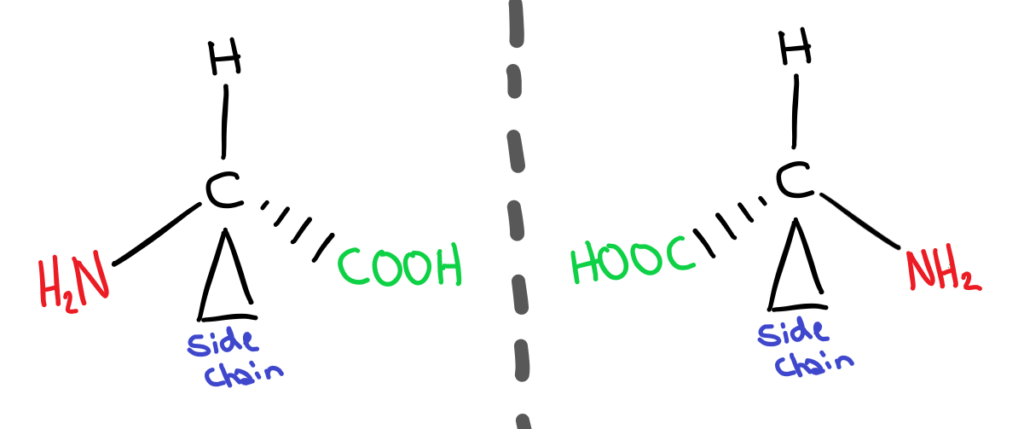
This is because amino acids have a carbon with 4 different functional groups bonded to it. These two different forms are called enantiomers. The naming system used to distinguish between the two isomers is the D&L system. In nature, amino acids only occur in one form, the L form.
It’s glycine of course ya spoon! Glycine isn’t chiral because it’s side chain is just a single hydrogen. Because of this, it doesn’t have 4 different functional groups and therefore cannot be chiral!
Why do we care?
Although the two enantiomers have the same physical and chemical properties, they have different biochemical properties. What does this mean? Different enantiomers interact and bond differently with other biochemical molecules. For example, the conformation of D amino acids would lead to different secondary structures of proteins, as the bond angles and therefore shape would be different.
Lipid isomers - Cis/Trans fats
As we’ve learnt in organic chem, double bonds can be either cis or trans. This is the same in fatty acids and it changes their properties.
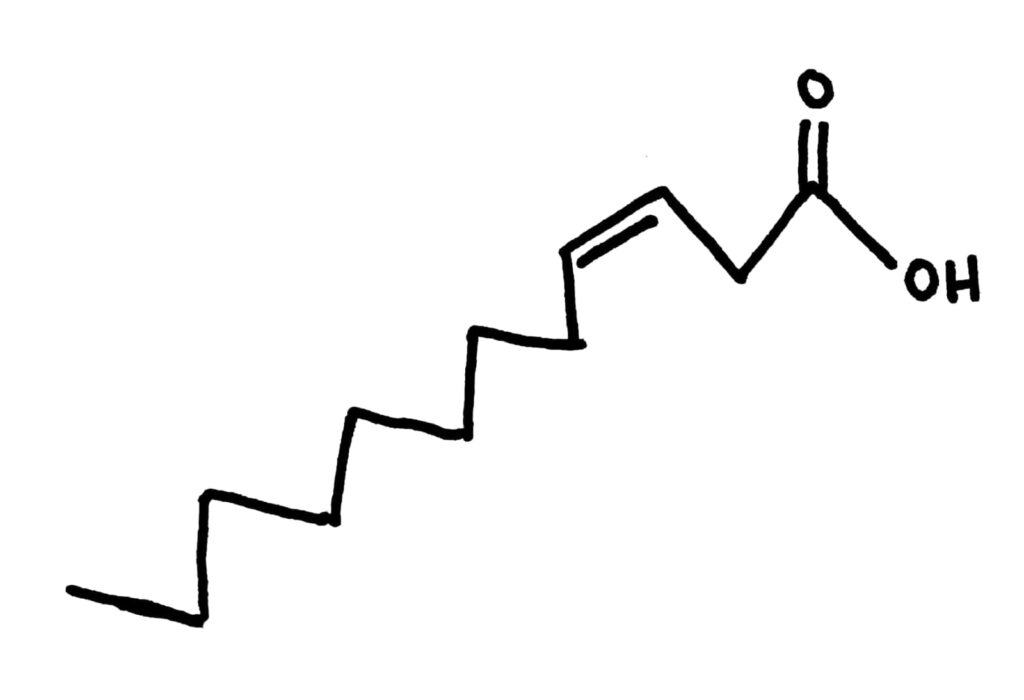
Cis fatty acids have a kink in their chain, giving them a kind of weird shape. This shape means that they don’t pack together as well, and usually have a low boiling point because of decreased LDFs compared to trans fatty acids. They’re usually liquid at room temperature. Most natural fats and oils are cis 
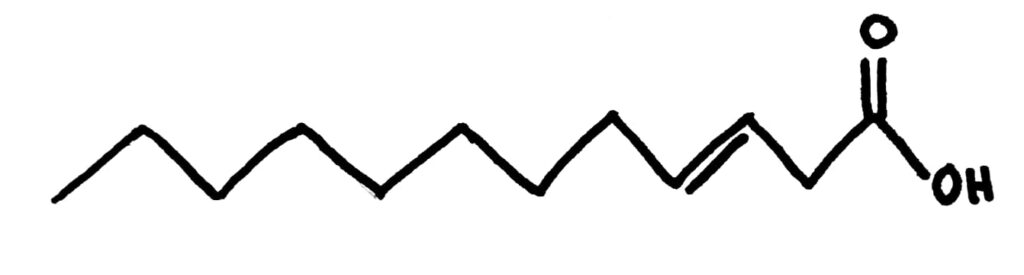


Trans fatty acids definitely pack much better than cis fatty acids, you can see it just from the number of them I managed to fit in the space where just 1 cis fatty acid could fit. This gives them a higher melting point because they also have increased LDFs compared to cis fatty acids and means they’re usually solids at room temperature 
Synthetic fats commonly have more trans double bonds (due to the hydrogenation process), and evidence points towards them giving people high blood pressure and heart disease. This is because they pack together and block arteries, causing high blood pressure.
All sugars have isomers because they contain chiral carbons.
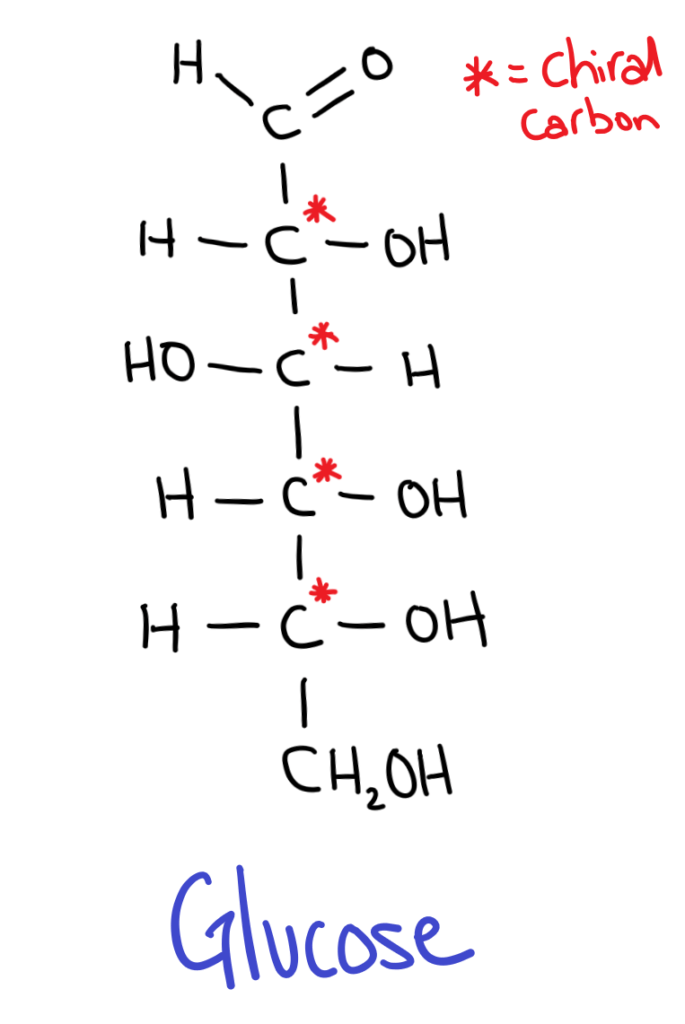
But wait, sugars have multiple chiral carbons so how can we decide whether it’s a D or L sugar?
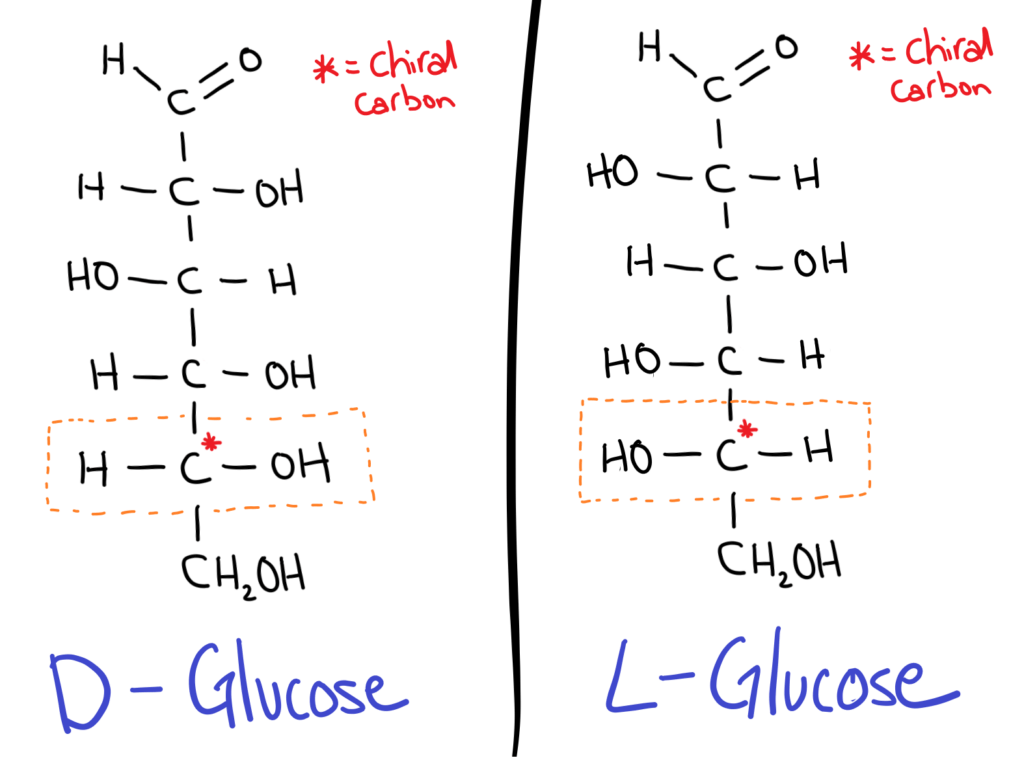
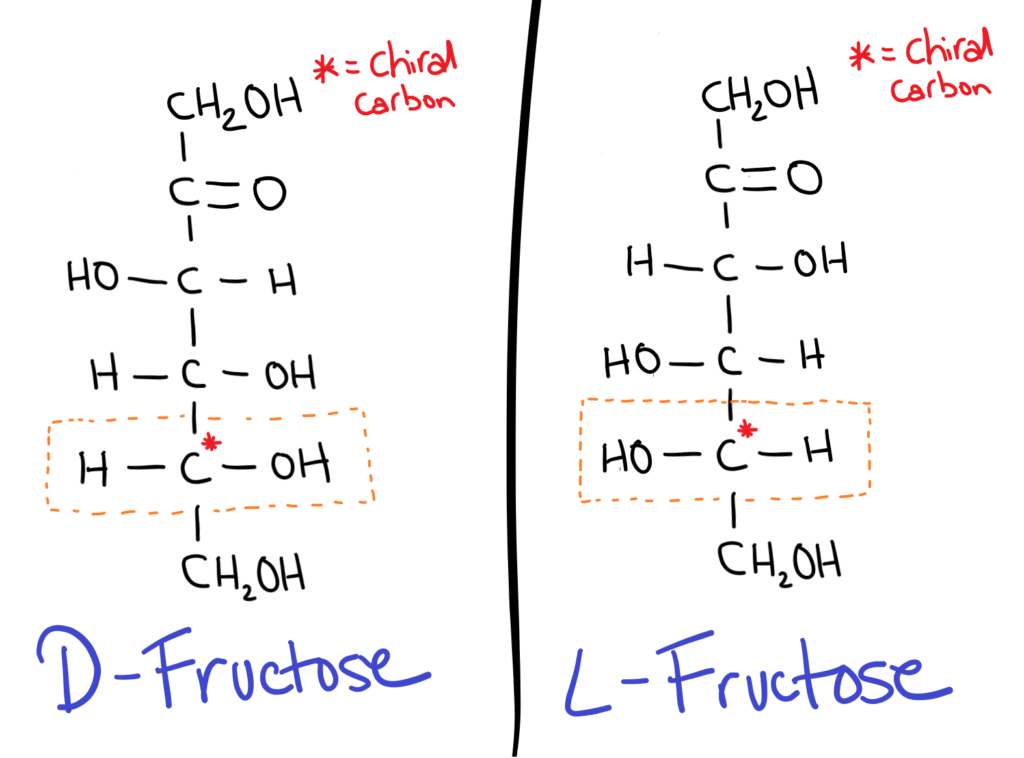
The trick is to take the chiral carbon furthest from the aldehyde or ketone group (the carbonyl). If the OH is on the RIGHT it’s a D sugar, if the OH is on the LEFT it’s an L sugar.
D sugars are most commonly found in nature.
It might seem kind of weird (it does for me at least) but in sugar isomers, the positions of all the OH groups are flipped!
Isomers in cyclic form
Don’t forget that sugars can be found in both the straight chain (above) and cyclic form! If you need to recap, go skim through B.4-Carbohydrates!
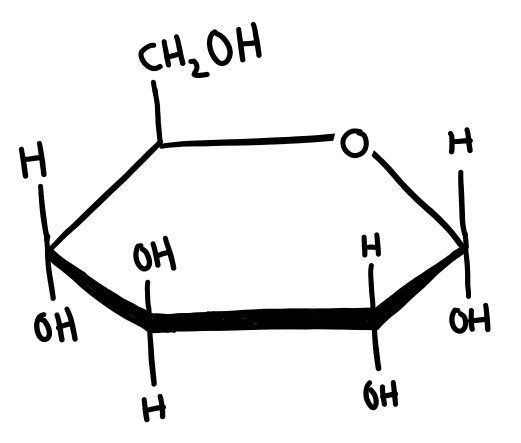
In cyclic form, sugars can have 2 isomers, α and β. Whether a sugar is α or β depends on the position of the OH group on carbon 1 (the carbon next to the Oxygen in the ring).
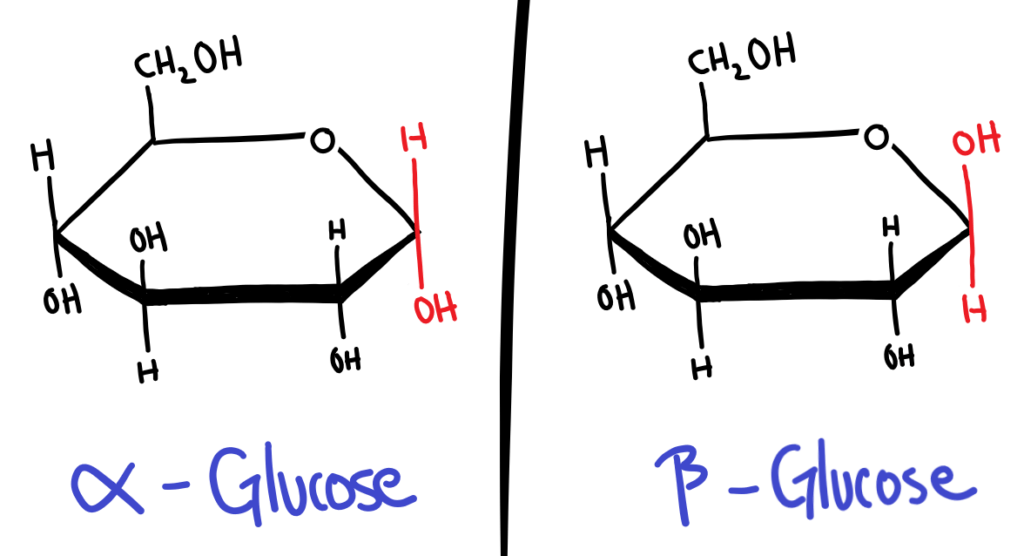
The easiest way I’ve found to memorise this is to assume the guy who first named this was on crack. α is for αbove the ring and it’s not, and β is for βelow the ring and it’s not.
Again, why do we care?
The conformation (α or β) of the sugar becomes especially important when the sugars bond together and polymerise. α-Glucose polymerises into starch, while β-Glucose polymerises into cellulose.
Starch
- An α-Glucose polymer
- Very tasty and energy rich 😁
Cellulose
- A β-Glucose polymer
- Not tasty or energy rich 😢
- Unless you’re a cow
- Great source of fibre
- Congratulations you are not constipated
Vision Chemistry
MOTIVATIONAL QUOTE OF THE DAY

You may have heard before that in our eyes, we have two different types of receptors; cones and rods. Cones detect colour while rods detect light intensity. In this topic we only focus on rods.
How do rods work?
In rods, there are 2 main components that are used in detecting light, retinal and opsin. Retinal is a biological molecule (that can be found in the data book!), and opsin is a protein.
Retinal has two different forms; 11-cis and all-trans:
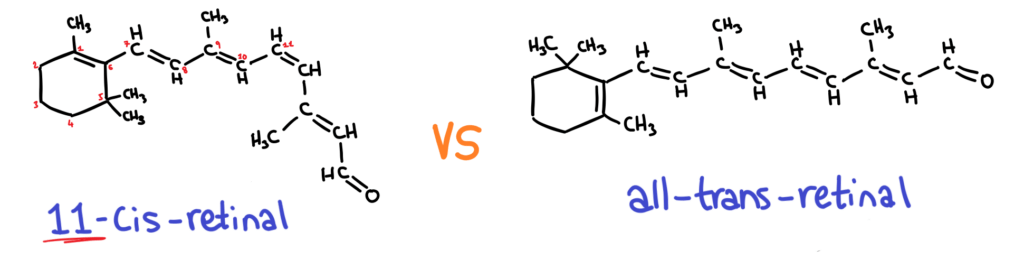
11-cis-retinal and opsin can bind to form rhodopsin, but all-trans retinal can’t!
Just for fun, here's rhodopsin:
So how does it work?
- When Rhodopsin is hit by light, the 11-cis-retinal changes into all-trans-retinal
- This change in conformation/shape triggers a nerve signal to the brain.
- The change in conformation of the retinal causes rhodopsin to break into all-trans-retinal and Opsin.
- Enzymes then convert all-trans-retinal back into 11-cis-retinal which then joins opsin to reform rhodopsin.
