WHAT ARE THE ALKALI METALS?
The Alkali Metals are metals from group 1 on the periodic table.
They are quite reactive. Hopefully your teacher has shown you the reaction between these metals and water. If not, head over to YouTube and search, there are hundreds of videos showing this! Of course I would make a video on this myself but being of 15 years of age I can’t get my hands on these reactive metals.
The Alkali metals are reactive because they have 1 electron in their outer shell that they are quite keen to get rid of. They want to get rid of it so they can become more stable, if an atom has a full outer shell then they will become very stable. The easiest way for the alkali metals to do this is to loose their 1 electron in their outer shell.
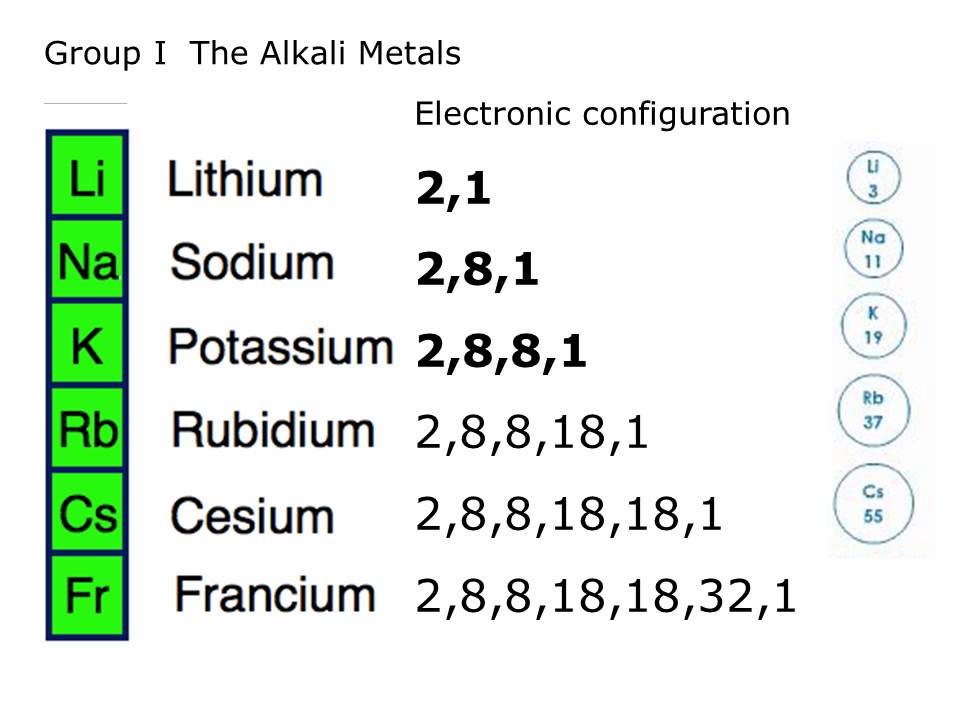
WHICH ONES ARE MORE REACTIVE?
With the Alkali Metals, unlike the Halogens, as you go down the metals are more reactive. Why you ask? As you go down the elements have more shells of electrons, each shell being further and further away from the nucleus. The nucleus’ positive charge keeps the electron from leaving. As you get further from the nucleus the attraction between the electron and the nucleus becomes weaker, making it much easier for the heavier elements to loose their extra electrons. Make sense? A bit like the further the paperclip from the magnet the weaker the attraction and the easier to pull it away.
REACTIONS WITH WATER

When they react with water, they do so by forming a hydroxide and giving off hydrogen gas. This reaction explains why these elements are called alkali metals, they react with water to form an alkali.
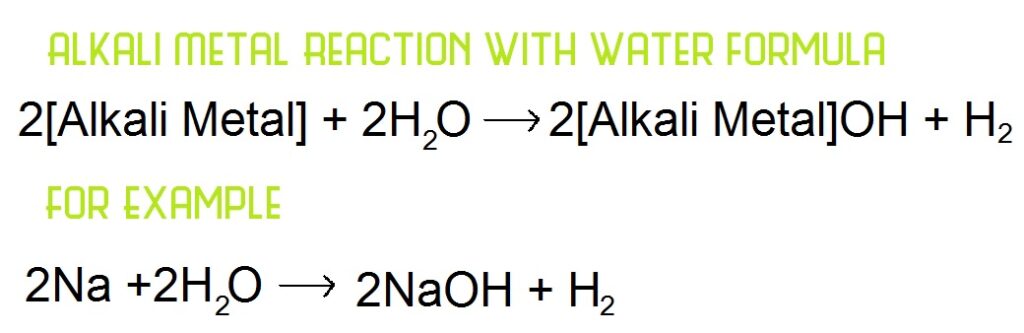
This reaction is used to show that these elements are all part of a family. This can be seen in the way they react, they all react in a similar way to create similar products, with increasing reactivity as you go down.
THAT'S ALL YOU NEED TO KNOW ABOUT ALKALI METALS!
I hope you found this useful, if not, feel free to let me know why and how I can improve in the comments or drop me an email. I’ll try to reply as soon as possible!