8.1 - Acid Base Theories
The Basics
(no pun intended)
In your previous studies you were probably told that acids are compounds that form H+ in solution, and that bases (or alkalis) are compounds that form OH-. In IB, whether you’re SL or HL, you’ll see why this isn’t 100% accurate
First things first: the H+ ion is a scam
H+ is a simplification of what actually happens. The H+ ion doesn’t exist for 2 reasons:
- H+ would be so reactive it would (and does) immediately react with whatever it around
- Water doesn’t just spontaneously break into H+ and OH- because it is a very stable molecule.
What really happens is that two water molecules bump into each other and a hydrogen (commonly referred to as a proton) is transfered, forming H3O+ and OH-.
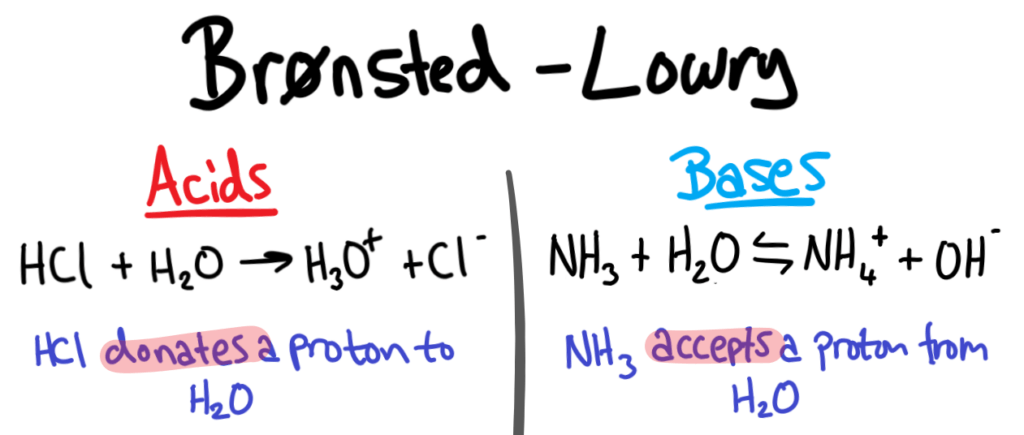
Brønsted-Lowry Acids and Bases
The Brønsted-Lowry theory of acids and bases defines acids and bases as the following:
- Acids are PROTON DONORS
- Bases are PROTON ACCEPTORS
When the water molecule and acid collide, a proton is transferred
- H2O and HCl collide
- The electron pair forms a dative/coordinate bond with the hydrogen
- The chlorine leaves with hydrogen’s electron to form Cl-
- The positive charge is transferred to the oxygen
- Oxygen has used two electrons (lone pair) to form the bond with hydrogen
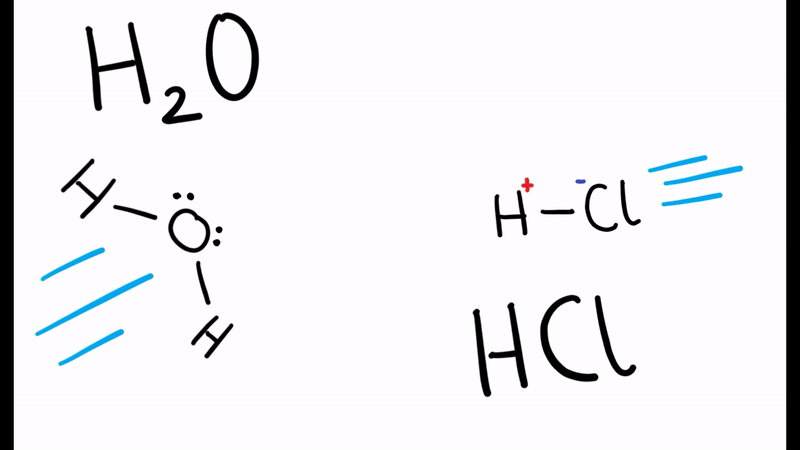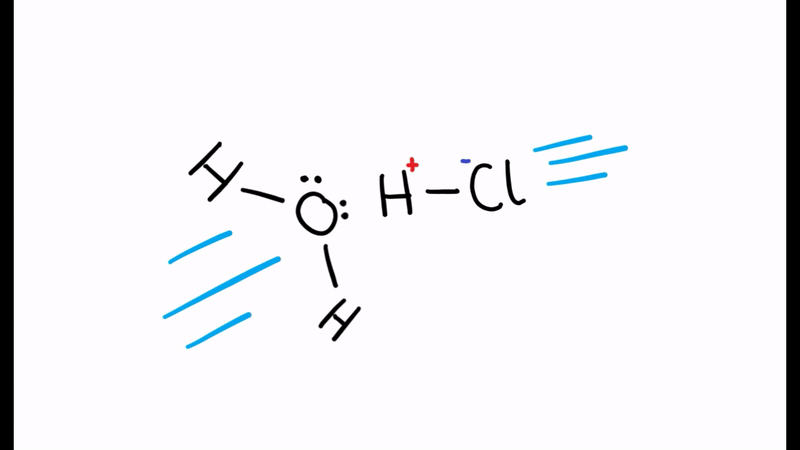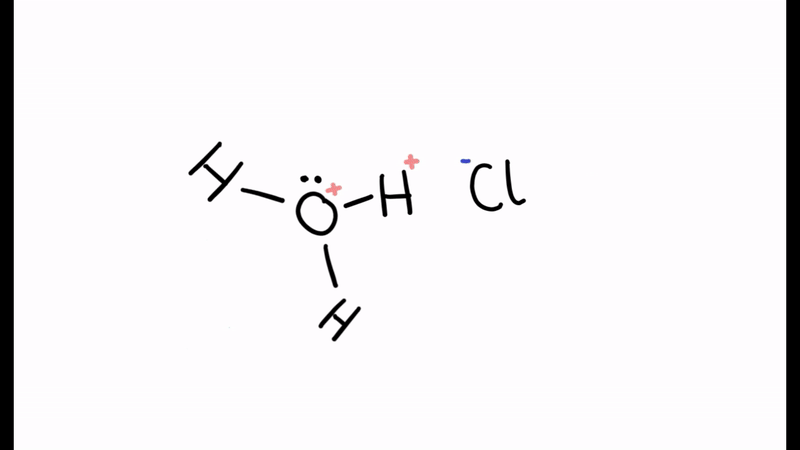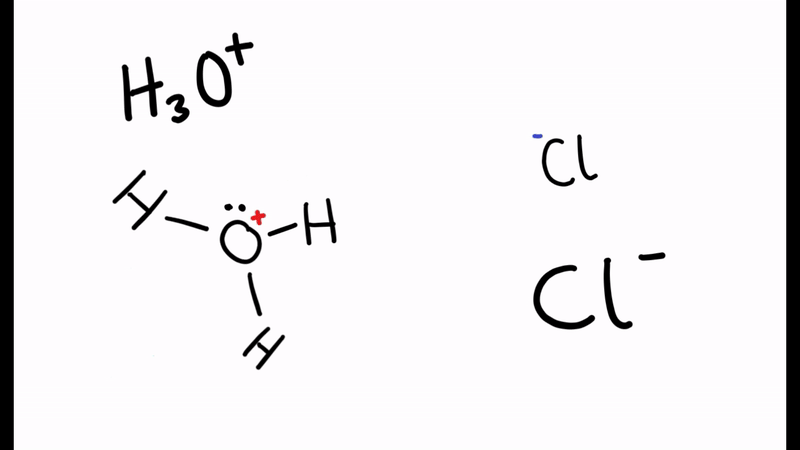
According to the Brønsted-Lowry theory, in the reaction below which of the reactants is the acid and which is the base?
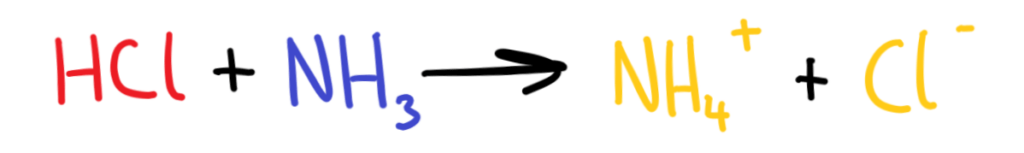
HCl is the acid and NH3 is the base.
HCl donates a proton to NH3 to form NH4+ (proton donor = acid)
NH3 accepts a proton from HCl to form NH4+ (proton acceptor = base)
Conjugate Acid & Base Pairs
Conjugate acid & base pairs are a pair of two species (molecules) that differ from each other by a single proton (H+)
Click on settings and change the test to answering in ‘English’ only for it to work 🙂